Made O'Meter
Discover where a brand or product originates
upload
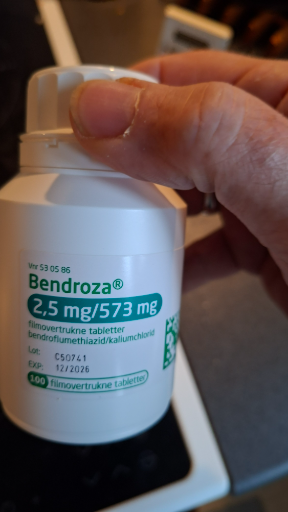
Bendroza® – bendroflumethiazid + kaliumchlorid tabletter
ownerTeva Pharmaceutical Industries Ltd. (Israel)
originIsrael
manufacturedmultipleCountries
Identification and composition:
- The pictured product is Bendroza®, a combination tablet containing bendroflumethiazide and potassium chloride (the packaging shows 2,5 mg / 573 mg). (pro.medicin.dk)
Marketing and local distribution:
- Bendroza® is marketed under the TEVA label in Denmark and appears in Danish medicine information services and pharmacy listings as Bendroza®, komb., containing bendroflumethiazide and kaliumchlorid. (apoteket.dk)
Manufacturing:
- Teva is a multinational pharmaceutical manufacturer that operates a global production network with many manufacturing sites across multiple countries; specific pack-level manufacturing site for this bottle is not shown in the photo, so the product should be considered produced in multiple countries within Teva’s global network. (tevapharm.dk)
Brand origin and ownership:
- The Bendroza® product is a TEVA-branded generic medicine. The brand/company behind Bendroza® is Teva Pharmaceutical Industries Ltd., a global pharmaceutical company originally founded and headquartered in Israel; Teva remains the ultimate owner. Teva operates through national subsidiaries (for example Teva Denmark A/S for the Danish market). (tevapharm.dk)
Notes and confidence:
- Identification confidence: high for product identity and active ingredients (clear Danish label text: "bendroflumethiazid/kaliumchlorid" and brand Bendroza®). Manufacturer site (country) is not printed on the visible part of the photo; Teva’s global manufacturing footprint means exact country of manufacture cannot be reliably determined from this image alone. (pro.medicin.dk)
Report a bug/Feedback
disclaimer
poweredBy